The concept of organ banking has long been a tantalizing prospect in medical science—a future where replacement organs are readily available, eliminating transplant waitlists and compatibility issues. With the convergence of 3D bioprinting and cryopreservation techniques, this vision is inching closer to reality. The marriage of these two technologies could revolutionize how we approach organ shortages, long-term storage, and even the ethics of transplantation.
At the heart of this breakthrough is 3D bioprinting, a process that layers living cells, biomaterials, and growth factors to construct functional human tissues. Unlike traditional 3D printing, which uses plastics or metals, bioprinting deals with the delicate balance of keeping cells alive while structuring them into complex organ-like formations. Researchers have already succeeded in printing skin, cartilage, and even rudimentary versions of kidneys and hearts. But the true challenge lies not just in creating these structures but in preserving them for future use.
This is where cryopreservation enters the picture. The science of freezing biological material without damaging it has been around for decades, famously used in sperm and egg storage. However, scaling this up to entire organs has been fraught with difficulties. Ice crystals form during freezing, rupturing cells and rendering tissues unusable. Recent advancements in cryoprotectants—chemicals that prevent ice formation—have begun to change that. Vitrification, a process that turns tissues into a glass-like state rather than freezing them, shows particular promise for preserving bioprinted organs.
Combining these technologies opens the door to an organ banking era. Imagine a scenario where hospitals maintain inventories of bioprinted organs, frozen and ready for revival when a patient needs them. This would drastically reduce the time-sensitive pressures of live organ transplants, where organs often degrade before reaching recipients. It could also democratize access to transplants, as organs could be stockpiled in advance rather than relying on unpredictable donor availability.
Yet, the path forward is not without hurdles. The intricacies of reviving cryopreserved organs remain a significant obstacle. While small tissue samples can be successfully thawed, larger structures like kidneys or livers suffer damage during the rewarming process. Uneven heating leads to cracks and cell death, rendering the organ nonviable. Researchers are experimenting with nanoparticles and electromagnetic fields to enable uniform warming, but these methods are still in experimental stages.
Ethical questions also loom large. If organs can be banked, who gets priority access? Will this technology be available only to those who can afford it, exacerbating healthcare disparities? And what are the implications of mass-producing human tissues—could it lead to commodification of the human body? These are not just theoretical concerns but pressing issues that will shape how this technology is regulated and deployed.
Despite these challenges, the progress is undeniable. Several biotech startups are already racing to commercialize aspects of this technology, focusing first on simpler tissues like skin grafts and corneas. Government agencies and academic institutions are pouring funding into research, recognizing the transformative potential. Clinical trials for bioprinted tissues are underway, and while full organs may still be years away, the foundational science is advancing at an unprecedented pace.
The implications extend beyond transplantation. Drug testing could be revolutionized by banks of cryopreserved, bioprinted human tissues, providing more accurate models than animal testing. Burn victims could receive instant skin grafts without painful harvests from their own bodies. The military is even exploring the technology for battlefield medicine, where rapid access to tissues could save lives in remote locations.
As with any medical breakthrough, the journey from lab to clinic will be long and fraught with setbacks. But the convergence of 3D bioprinting and cryopreservation represents one of the most promising frontiers in modern medicine. The dream of organ banking—once the stuff of science fiction—is now a tangible goal, poised to redefine the boundaries of life-saving treatments.

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025
By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025
By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025
By /Jul 2, 2025
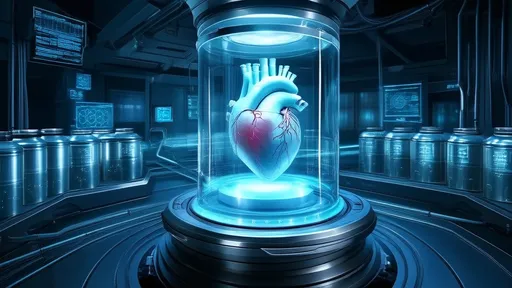
By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025

By /Jul 2, 2025